Allocell®
Allograft Fiber Bone Matrix

MF
The implant-ready fibers require no prior preparation, offering an efficient solution for the management of small bony voids. The design makes them well-suited for minimally invasive techniques and procedures performed through limited surgical access.
AF
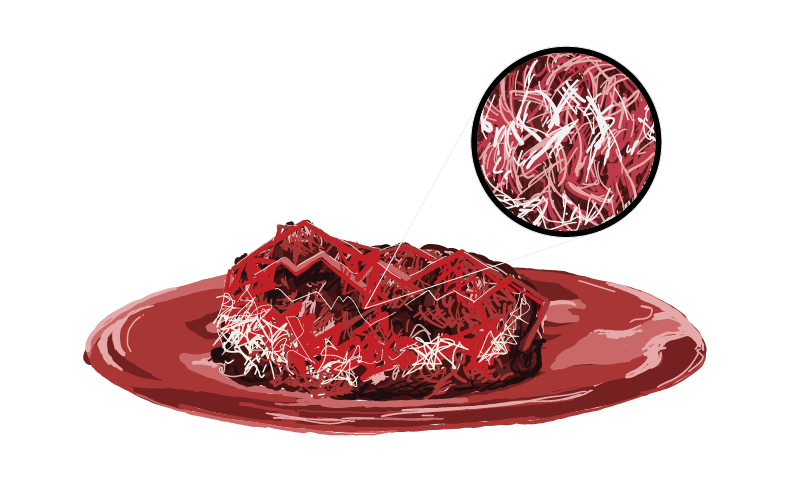
The graft material is designed to address large bony voids and high-volume procedural requirements, making it well-suited for use in posterolateral gutters as well as within cages featuring large graft windows.
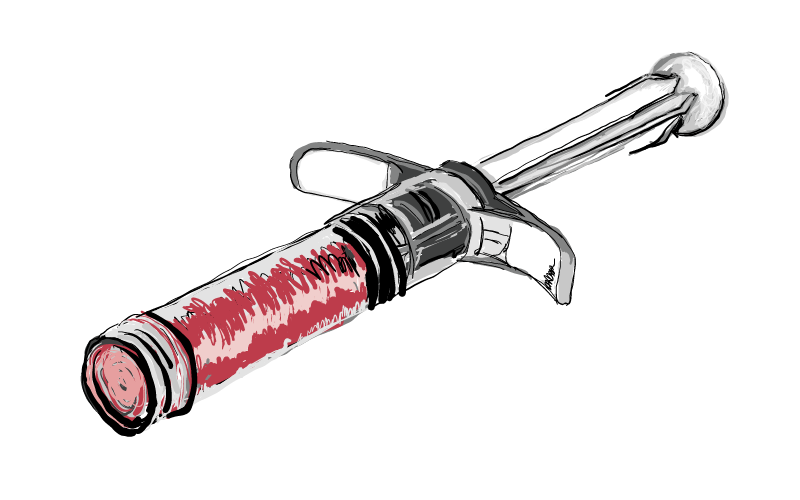
FLOWABLE
The 100% bone fiber DBM provides inherent osteoinductive properties while supporting bone growth through an osteoconductive scaffold. Convenient open-barrel syringe for ease of preparation and precise placement.

STRIP
The material provides an osteoconductive scaffold with growth factors and can be combined with autograft or other biologics at the surgeon’s discretion. It is easily hydrated and moldable to the desired shape, with a sterile assurance level of 10⁻⁶ to minimize infection risk.
Introducing
Allocell® LIVE
Our new viable bone matrix allograft representing the latest advancement in cellular bone technology. The graft offers an exceptional alternative to autograft through its osteoconductive, osteoinductive, and osteogenic potential. Our proprietary processing methods protect the native elements of bone, including growth factors and viable cells, while our advanced Clearant® fiber technology creates exceptional handling characteristics.
Osteoinductive Potential
Patented demineralization procedure effectively exposes and preserves endogenous growth factors within cortical tissue.
Osteogenic Potential
Proprietary processing removes blood and marrow while retaining viable cells bound to the native bone structures.
Osteoconductive
Interconnected fiber matrix and trabecular cancellous structure create a robust scaffold.
Fiber Bone Matrix
Allocell® AF and MF products are manufactured utilizing a process that provides for graft consistency in hydration, moldability, osteoconductive and osteoinductive potential. The Allocell process provides for a graft with optimal fluid retention and irrigation resistance.
Precise handling with the 2 options of fibers, Allocell® AF and MF allow the surgeon the capability for precise placement as well as retention at the graft site.
Our Allocell® demineralized bone fibers are made from 100% human tissue and designed for surgeons who want maximum osteoconductive and osteoinductive potential with fewer post-surgical complications.
Allocell® is a second-generation allograft with proprietary narrow and wide elongated inductive fibers interconnected in a weave that provides more continuous surface area for cellular attachment and proliferation


Want more information on Allocell®? Submit the quick contact form and our product specialists will get back to you right away!

